What Is the Average Atomic Mass of Chromium
The average atomic mass of chromium is 5200 u. Atomic Mass of Chromium.
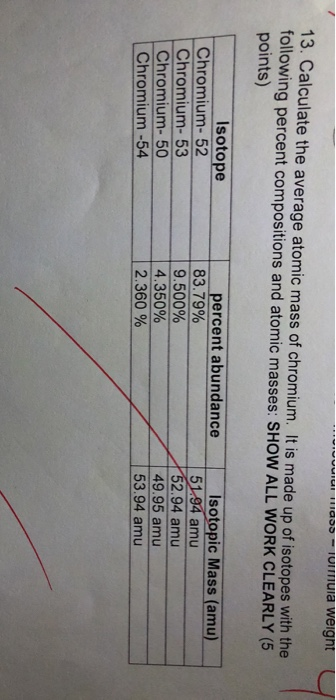
Solved 13 Calculate The Average Atomic Mass Of Chromium It Chegg Com
Cr-52 is the answer.

. Given the average atomic mass of an element on the periodic table and the percent natural abundance of each isotope calculate the identity of the unknown isotopeAtomic mass of chromium is 51996 amu. What is the mass of 30 mol of chromium. Naturally occurring chromium 24 Cr is composed of four stable isotopes.
The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element. 50 Cr is suspected of decaying by β β to 50 Ti with a half-life of more than 1810 17 years. Isotope Percentage Cr-50 43 Cr-52 838 Cr-53 95 Cr-54 24 A.
Based on the data below what is the average atomic mass of chromium. What is the mass of 300 mol of chromium. The atomic mass is the mass of an atom.
Example 2 Calculate the average atomic mass of chromium. Not in percents Isotope Mass amu Relative Abundance Chromium 50 49946 0043500 Chromium 52 51941 083800 Chromium 53 52941 0095000 Chromium 54 53939 0023500 7. Cr-52 because its mass number is closest to the actual atomic mass of Chromium 51996.
The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element. Given the average atomic mass of an element on the periodic table and the percent natural abundance of each isotope calculate the identity of the unknown isotopeatomic mass of chromium is 51996 amu chromium-. What is the average atomic mass.
The atomic mass is the mass of an atom. 435 with a mass of 4995 amu. It solves for total mass of a molecular formula average molecular weight.
4345 chromium-52 8379 chromium-53 950 chromium-54 2365. 950 with a mass of 5294 amu. 434 of 50Cr with an atomic weight of 499460 amu.
8379 with a mass of 5194 amu. It is made up of isotopes with the following percent compositions and atomic masses. Meanwhile report isotope fractionation of chromium during chromate reduction resulting in δ53 Cr SRM979 values in groundwater samples as high as 58 or Ar Cr 519982 which is outside the current range of.
950 of 53Cr with an atomic weight of 529407 amu. Calculate the average atomic mass of chromium. We dont have brackets implemented yet so you will need to unpack any bracketed expressions.
What is the average atomic mass of chromium. Click here to get an answer to your question The average atomic mass of chromium is 5200 amu. The average atomic mass sometimes called atomic weight of an element is the weighted average mass of the atoms in a naturally occurring sample of the element.
Standard atomic weight Ar standardCr 519961 6 view. Chromium is a meta element. They dont affect the weight anyhow.
53938 878 3 0023 65 7 In 1983 the Commission recommended the standard atomic weight of chromium to four decimal places Ar Cr 519961 6. Mechanical Engineering questions and answers. As always you must show all work show all units observe significant figures and box your final answers.
8379 of 52Cr with an atomic weight of 519405 amu. The average atomic mass of chromium is 5200 amu. Atomic mass of Chromium is 519961 u.
435 with a mass of 4995 amu. 8379 with a mass of 5194 amu. The average atomic mass of chromium is 51996 amu.
The average atomic mass of copper is 6355 amu. And 236 with a mass of 5394 amu. Atomic mass of Chromium is 519961 u.
From there we break the formula for Chromium chloride - 3 into parts - a Chromium atom a Chlorine atom etc. 950 with a mass of 5294 amu. Average masses are generally expressed in unified atomic mass units u where 1 u is equal to exactly one-twelfth the mass of a neutral atom of carbon-12.
Atomic mass of Chromium is 519961 u. And 237 of 54Cr with an atomic weight of 539389 amu. If the only two isotopes of copper.
Atomic mass of it is 52. Note that each element may contain more isotopes. Calculate the average atomic mass of chromium.
What is the mass of 300 mol of chromium. Chromium has four naturally occurring isotopes. On the basis of these data confirm that the average atomic.
50 Cr 52 Cr 53 Cr and 54 Cr with 52 Cr being the most abundant 83789 natural abundance. Therefore this resulting atomic mass is calculated from naturally-occurring isotopes and their abundance. 236 with a mass of 5394 amu.
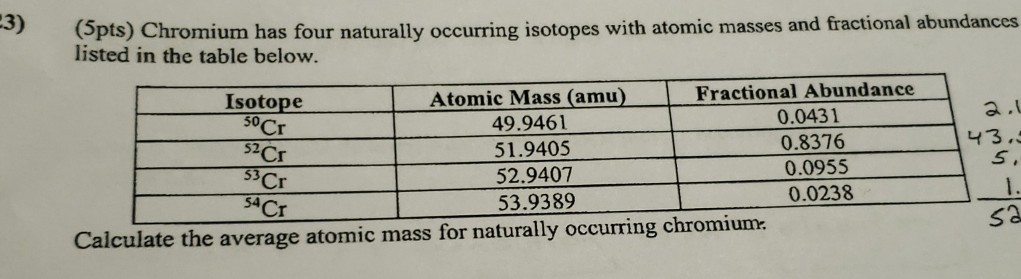
Solved 3 Pts Chromium Has Four Naturally Occurring Chegg Com
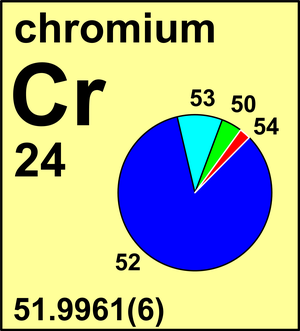
Atomic Weight Of Chromium Commission On Isotopic Abundances And Atomic Weights

I Really Need Someone To Explain How To Find The Average Atomic Mass The Gizmos Was Really Brainly Com
No comments for "What Is the Average Atomic Mass of Chromium"
Post a Comment